Abstract
Introduction. We report here the results of the EWALL-INO Phase 2 study (ClinicalTrials.gov: NCT03249870) evaluating the activity and safety of frontline inotuzumab ozogamicin (INO), an anti-CD22 antibody conjugated to calicheamicin, combined to low-intensity chemotherapy in 131 older patients with CD22+ Ph-negative BCP-ALL.
Methods. This single arm prospective study has been conducted in several European centers from the European Working group on adult ALL (EWALL). Eligibility criteria were patients aged ≥55 years, with an ECOG performance status ≤2, newly diagnosed with CD22+ (20% or more of positive blast cells) Ph-negative BCP-ALL without central nervous system (CNS) involvement. After a prephase including 5 days (D) of dexamethasone (DEX) 10mg per D and intrathecal (IT) methotrexate (MTX), the induction regimen was delivered in 2 parts. Induction part I (Ind1) consisted of one triple IT, vincristine (VCR) 2 mg (1 mg over 70 years of age) D1 D8 D15 D22 and DEX 20 mg D1D2 D8D9 D15D16 D22D23 combined with 3 injections of INO (0.8 mg/m² D1, 0.5 mg/m² D8 and D15). Induction part II (Ind2) was offered to patients in CR or CRp (CR with platelets <100 x 109/L) after Ind1 or as salvage therapy. Ind2 consisted of DEX 20mg D1D8, cyclophosphamide (CY) 300 mg/m² D1 to D3, one triple IT D2 and 2 injections of INO (0.5 mg/m² D1 and D8). Patients in CR/CRp were programmed to receive 6 blocks of consolidation (Ara-C 1.5g/m²/12h adapted to renal clearance D1D2 and DEX 10mg/12h D1D2, cycles 1 and 4; MTX 1.5 g/m² over 24h D1, VCR 1 or 2 mg D1, one triple IT D2 and 6-mercaptopurin (6-MP) D1 to D7, cycles 2 and 5; CY 500 mg/m² D1D2, VP16 75 mg/m² D1D2, one triple IT D2 and MTX 25 mg/m² D1, cycles 3 and 6) followed by a POMP maintenance (VCR, 6-MP, MTX, DEX) during 18 months. Allogeneic hematopoietic stem cell transplant (HSCT) was allowed after at least 3 blocks of consolidation at the discretion of the investigator. The primary endpoint of the study was overall survival (OS) with an expected 1-year OS rate of 70%. The evaluable population consisted of patients who received at least 1 dose of INO. Minimal residual disease (MRD) was assessed on bone marrow aspirates after Ind1 and Ind2 using quantification of clonal molecular rearrangements. The study was planned to recruit up to 130 patients. All patients provided informed consent.
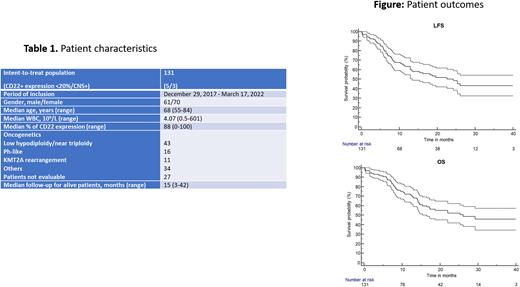
Results. Between 12/2017 and 03/2022, 6 screen failures occurred (including one Ph+ ALL) and 131 patients were enrolled and received at least one dose of INO. These 131 patients were considered for the intent-to-treat analysis. However, among them, 5 patients had CD22 expression <20% and 3 had CNS involvement. Patient characteristics are shown on Table 1.
After Ind1, the CR/CRp rate was 88.5% (116/131, 9 CRp) and 4/131 (3%) patients had died. MRD <10-4 was achieved in 56 of 97 (56.6%) CR/CRp tested patients. A total of 120 patients received Ind2 (115 CR/CRp patients, as 1 death in CR occurred between Ind1 and Ind2 and 5 patients received Ind2 after failure of Ind1) and 118 patients eventually achieved CR/CRp (112 CR, including 3 late CR after Ind2; 6 CRp) resulting in a CR/CRp rate of 90% (118/131) after Ind1 + Ind2. No patient died during Ind2. MRD <10-4 was achieved in 80 of 99 CR/CRp tested patients (80.8%) after Ind2. Overall, the full planned INO dosing was administered to 108 patients (82.5%).
With a median follow-up of 15 months (range 3-42), 29 patients relapsed and 50 patients died, including 29 deaths after relapse. Only 11/118 CR/CRp patients received an HSCT (9.4%). With a 1-year OS of 72.5% (95% CI, 64-81), the primary endpoint was reached. At 2 years, OS was 53.6% (95% CI, 44-64). Leukemia- free survival was 64.9% (95% CI, 56-74) at 1 year and 49.8% (95% CI, 40-61) at 2 years. (Figure 1)
A grade 3-4 liver toxicity was observed in 21 patients (16%), including 3 patients (2.2%) with transient sinusoidal obstruction syndrome (SOS), 2 during Ind1 and 1 after HSCT.
Conclusion. These results confirm that fractionated INO combined to low-intensity chemotherapy is a very active and well tolerated frontline therapy for older patients with CD22+ Ph-neg BCP-ALL, as previously reported in the MDACC INO/mini-HCVD study (Jabbour et al. Cancer 2019). An updated analysis will be provided at ASH meeting.
Disclosures
Chevallier:Abbvie: Honoraria; Incyte: Research Funding; Takeda: Honoraria; Pfizer: Research Funding; Jazz Pharmaceuticals: Honoraria. Doubek:Janssen: Honoraria; AstraZeneca: Consultancy, Honoraria, Research Funding; AbbVie: Honoraria; AOP Orphan: Consultancy, Research Funding. Huguet:novartis: Honoraria; incyte: Honoraria; bms: Honoraria; amgen: Honoraria; pfizer: Honoraria; jazz pharma: Honoraria. Lepretre:AbbVie, Roche, Amgen, AstraZeneca, Janssen, Beigene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Boissel:GILEAD: Honoraria; AMGEN: Honoraria; ARIAD/INCYTE: Honoraria; ASTELLAS: Honoraria; NOVARTIS: Honoraria; SERVIER: Honoraria. Rousselot:Bristol Myers Squibb: Consultancy; Pfizer: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Takeda: Consultancy; Novartis: Consultancy.
OffLabel Disclosure:
INOTUZUMAB in first line therapy for ALL
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal